
Close


In the vast landscape of pharmaceuticals, there exists a category of drugs that serves a unique and often overlooked purpose – orphan drugs. These medications are specifically developed to treat rare diseases, offering a ray of hope to those facing the challenges of conditions that affect only a small portion of the population. In this blog post, we’ll explore the world of orphan drugs, delving into their significance, development process, and the impact they have on individuals with rare diseases.
An orphan drug is a pharmaceutical agent developed to treat a rare medical condition, often referred to as an orphan disease. The term \”orphan\” is used because these diseases typically affect a small number of people, making them financially unattractive for pharmaceutical companies to invest in compared to more prevalent conditions. These drugs aim to address the unmet medical needs of individuals facing rare diseases, providing hope where treatment options are limited or non-existent.
Orphan drugs are pharmaceuticals developed to address rare medical conditions, and their classification as such varies by country. In the United States, the FDA considers diseases affecting fewer than 200,000 individuals as rare, while the European Union defines rarity as affecting fewer than 1 in 2,000 people. In Japan, a disease is typically rare if it affects fewer than 50,000 people. Orphan drug designation requires demonstrating a significant clinical benefit, often with considerations for the absence of effective treatments. These criteria encourage the development of treatments for rare diseases by providing incentives such as market exclusivity and financial benefits.
The Orphan Drug Act (ODA) was enacted in the United States in 1983 to encourage the development of drugs for rare diseases. This legislation provides incentives to pharmaceutical companies, such as tax credits, grants, and market exclusivity, to offset the financial challenges associated with developing drugs for small patient populations. The ODA has been instrumental in fostering innovation and driving research in the field of rare diseases, leading to the discovery and development of numerous orphan drugs.
Developing orphan drugs for rare diseases involves a rigorous and specialized process that differs from the development of drugs for more common conditions. The limited patient pool and unique nature of rare diseases present distinct challenges to researchers and pharmaceutical companies. The process typically includes:
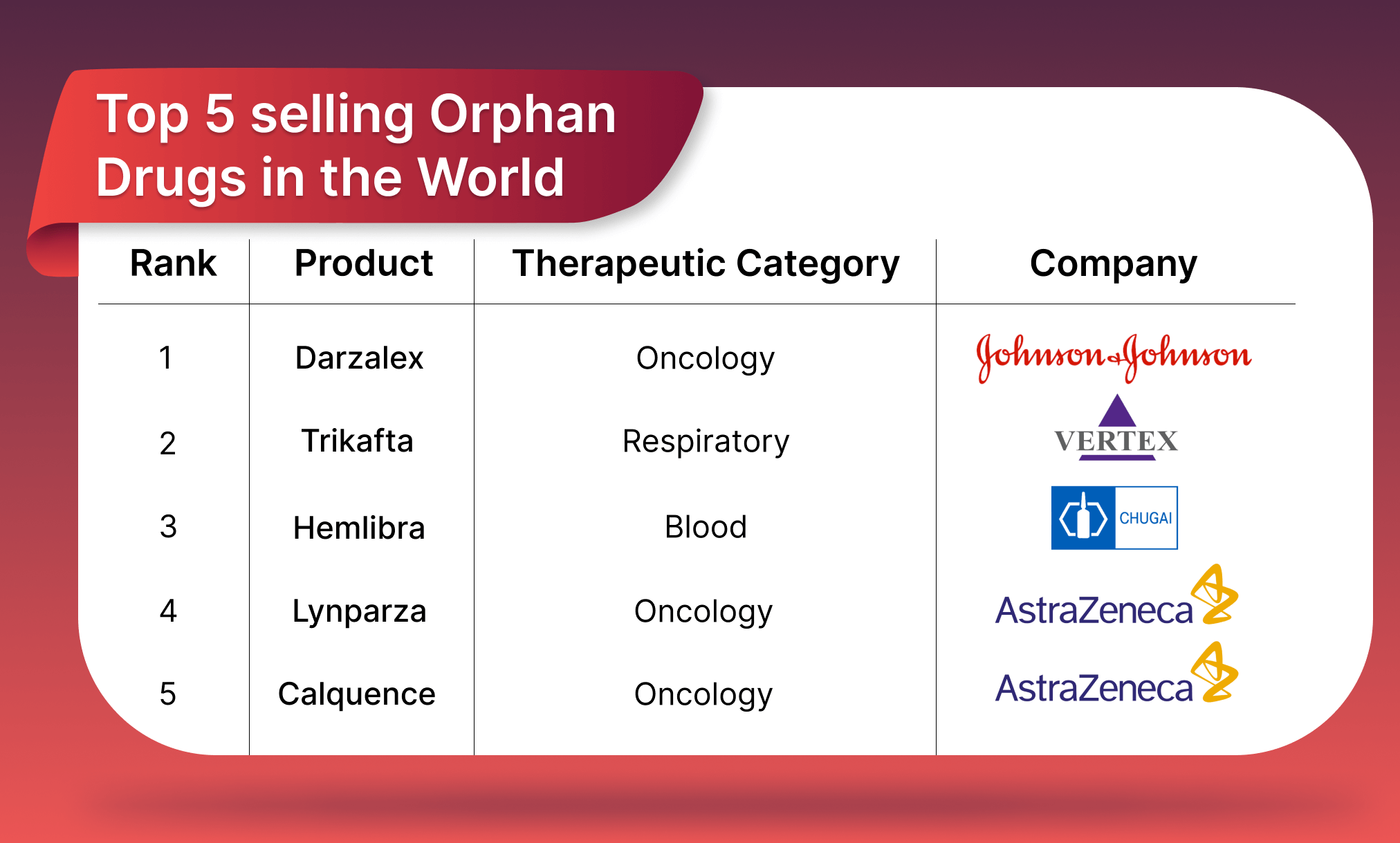
Source: >>
Orphan drugs play a crucial role in improving the quality of life for individuals with rare diseases. They offer a lifeline to patients who may have previously faced limited or no treatment options. By addressing unmet medical needs, orphan drugs can slow disease progression, alleviate symptoms, and, in some cases, provide a cure.
The need for increased awareness, continued research, and global collaboration remains paramount to advance the field of orphan drug development.
In conclusion, orphan drugs play a pivotal role in addressing the unmet medical needs of individuals with rare diseases, offering essential treatment options where none previously existed. Through targeted research, innovative development processes, and regulatory incentives, these medications represent a substantial advancement in healthcare. Despite the challenges integral to their development, orphan drugs mitigate the impact of rare diseases, demonstrating the commitment of the pharmaceutical industry to improve patient outcomes and reduce suffering, underscoring the importance of continued investment and collaboration in the field of rare disease treatment.
Maintaining a competitive edge in the orphan drugs sector necessitates a deep understanding of the advancing technological landscape. We guide businesses in staying ahead through strategic technological insights. Visit our Technology Landscape service page to shape your success in the dynamic field of Orphan Drugs.